Why We Selected Zinc & Yellow Chromate Plating For Our Stand-Off MPV Bracket
Several product reviewers have asked us why we choose zinc and yellow chromate plating for our Stand-Off MPV Bracket.
We Base Our Decision On The Following
We wanted to offer our Stand-Off MPV Bracket in carbon steel with a protective coating as well as stainless steel since coated carbon steel is more economical than stainless steel and easier to install fasteners through.
Hot-Dip Galvanizing Did Not Work
Our first inclination was to have the brackets hot-dip galvanize plated per ASTM A153 Class B-2 which is the construction industry standard for hot-dip galvanizing of metal components used in exterior wall applications. We delivered two dozen samples to a local galvanizing company for trials. They tried several methods, but none resulted in the threaded parts of our brackets being able to be threaded together.
Our consultant explained to our prototype manufacturer and ourselves that the hot-dip galvanizing material in molten form can still contain small bits of metal that are not melted and that when the parts are removed from the dipping tanks, the melted metal can still run and form drips which can garden on that state on the parts.
Hiring A Coatings Consultant to Identify Coatings with Longevity
We then hired a coatings consultant to help us identify coatings that would result in the same longevity as hot-dip galvanized plating.
They explain that hot-dip galvanizing is a process by which a zinc-rich compound is applied to the surfaces of the steel and acts as a sacrificial layer to protect the carbon steel from corroding. The higher the zinc content of the coating and the thicker the coating, the longer it would last. Zinc is the sacrificial element of this type of coating compound.
Hot-dip galvanizing, in accordance with ASTM A153 Class B-2, requires a 98% zinc content with 1.5 ounces of zinc total for plating both sides of the steel element. This translates to 2.2 total mils thickness of the plating and 1.1 mils thickness per side.
In searching for a plating that will result in a 98% zinc content and 1.2 mils thick, they recommend mechanical zinc plating or what is commonly called in the plating industry, “barrel plating”.
Refer to The AGA Paper on Galvanizing
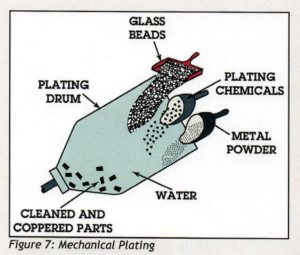 They referred us to the American Galvanizing Association’s paper entitled “Zinc Coatings: A Comparative Analysis of Process and Performance Characteristics. Page 10 of the document explains the Mechanical Plating process. The industry standard for mechanical plating is ASTM B633.
They referred us to the American Galvanizing Association’s paper entitled “Zinc Coatings: A Comparative Analysis of Process and Performance Characteristics. Page 10 of the document explains the Mechanical Plating process. The industry standard for mechanical plating is ASTM B633.
Within this standard Table 1 cites thicknesses required based on service conditions. Our consultant recommended the thickness Class of SC 4 ( very severe ). The Classification Number and Conversion Coating Suffix for this class are Fe/Zn 25 which requires a coating thickness of 25 microns which converts to .98 mils.
The finish type they recommended is Type II which is for colored chromate coatings. This requires withstanding a minimum of 96 hours in a salt spray apparatus.
We asked what this converted to in years, and they informed us that there is no industry standard conversion for hours in a salt spray tank and years of exposure. They referred us again to the attached paper from the American Galvanizing Association for durability assessment.
Their basis for assessing durability is what they call “Time To First Maintenance”. This is based on real-world exposures and assessment of the effects of the exposures.
What is “Time to First Maintenance”?
They define first maintenance as the time to 5% rusting of the steel surface. They offer Figure 5 on page 5 for the results of their research. Based on a 1 mil thick coating of a 98% zinc content coating, the “time to first maintenance” is 25 years for a suburban exposure. They also state that the results are linear based on the thickness of the plating and years of exposure and express this in Figure 5. Based on this figure hot-dip galvanizing ( 1.1 mils of 98% zinc content plating ) will result in 27.5 years ( 25 x 1.1 ) to “First Maintenance”.
Mechanical plating per ASTM B633 Fe/Zn 25 Type II results in 24.5 years ( 25 x .98 ) to “First Maintenance”.
Trial Samples of Mechanical Plating Sent to Local Plating Company
We sent a few dozen samples to a local plating company for trials and found that the threaded parts would not thread together completely. This was not near as bad as the inability to thread after the hot-dip galvanizing, though, so we had some hope that this could be worked out.
Between our coating consultant and the company that produced our prototypes, they came up with the recommendation that we increase the diameter of the female threaded part of our bracket by the thickness of the coating on each threaded part and it should work as mechanical plating results in a plating that does not have drips pieces of un-melted metal attached to the parts due to the plating process.
We had our prototype company increase the diameter of the female threaded part of our bracket by 2 one-thousandths of one inch ( 2 mills ). This resulted in the threaded parts being able to be completely threaded together.
An Acceptable Plating per Construction Industry Standard
At this point, we felt that we had an acceptable plating equal to hot-dip galvanizing per the construction industry standard ( ASTM A153 Class B-2 ). But we wanted to research what our competition was offering for corrosion resistance.
What is Our Competition Offering for Corrosion Resistance?
We found that they offer products with Continuous Sheet Galvanizing per ASTM A653 and ASTM A1046.
We looked into both of them and found that the components of their products were cut from sheets of steel that were hot-dipped galvanized. Our concern was the cut edges would not have the protective coating.
Our coating consultant told us that the companies that do this type of plating claim that the cutting ( shearing ) of the plated metal resulted in some of the coating being dragged across the cut which would offer protection and that over the years, more coating would migrate to the cut edge.
This did not sound logical to us so we ask our coating consulting about this. They agree with us and point out that this is not addressed by the American Galvanizing Association. We can only surmise the reason that it is not.
Issues with Plating Used by Our Competitors
Another issue we found with the plating used by our competitors on their steel components was the thickness of the plating and the zinc content. Both will play a role in determining “Time To First Maintenance”.
The competitors that offer components plated per ASTM A643 use Coating Grades of G 40 and G 90. These coatings require a 98% zinc content plating at .36 mils and .81 mils respectively
( See ASTM 6531 Table 1 or Table 1 on page 6 of the American Galvanizers Association paper ).
Using the “Time To First Maintenance” Chart in the American Galvanizers Association paper, the “Time To First Maintenance” for G 40 and G 90 galvanizing is 9 years ( 25 years x .36 mils ) and 20.25 years ( 25 years x .81 mils ).
The competitor that offers components plated per ASTM A 1046 uses Coating Grade ZM 40. The minimum thickness required is standard for this coating is .36 mils
( See ASTM A10461 Table 12 ).
The standard also states a variety of metal compositions for the hot-dipping baths and requires the user to specify the composition of the bath. This competitor states that they use a coating compound with 91% zinc. Based on the “Time To First Maintenance” Chart in the AGA document, this coating would result in a “Time To First Maintenance” of 8.36 years ( 25 years x .9286 ( percentage of zinc content versus ASTM A653 plating ) x .36 mils ).
In Conclusion The Basis of Our Selection of Zinc & Yellow Chrome Plating
Based on this, we chose zinc & yellow chromate plating per ASTM B633 Class Fe/ZN 25 Type II for the protective coating on the steel components of our Stand-Off MPV Bracket. We feel this offers the closest grade of protection to hot-dip galvanizing per ASTM A153.
Note 1: ASTM does not allow reproduction of their printed documents. Please refer to a copy of the document.
Note 2: ASTM A1046 Table 1 requires a coating minimum of .40 oz / SF total for both sides of the plating compound which is .20 oz / SF per side. This translates to .36 mils or 9.1 microns
Technical Note 01 – December 2021

 Len Anastasi has been working in the construction industry for over 30 years in masonry, waterproofing and restoration work.
He currently owns EXO-TEC Manufacturing, Inc., EXO-TEC Solutions, Inc. and EXO-TEC Consulting, Inc.
In his construction and consulting work, he has performed inspections and repairs on over 300 buildings.
Len has given expert testimony in trials and reviews on dozens of legal cases.
He is a member of ASTM’s E 06 Committee, the Boston Society of Architects Building Enclosure Council, Air Barrier Association of America, the Construction Specifications Institute, and the International Concrete Repair Institute.
Book Len for your Next Event!
Len Anastasi has been working in the construction industry for over 30 years in masonry, waterproofing and restoration work.
He currently owns EXO-TEC Manufacturing, Inc., EXO-TEC Solutions, Inc. and EXO-TEC Consulting, Inc.
In his construction and consulting work, he has performed inspections and repairs on over 300 buildings.
Len has given expert testimony in trials and reviews on dozens of legal cases.
He is a member of ASTM’s E 06 Committee, the Boston Society of Architects Building Enclosure Council, Air Barrier Association of America, the Construction Specifications Institute, and the International Concrete Repair Institute.
Book Len for your Next Event! 